


Lead-acid batteries, which are used in General Motors' EV1 electric car, have a limited 70-mile range and run anemically, if at all, in cold weather. In fact, GM only offers the EV1 in sunny climates such as Arizona and southern California.
Use of lead-acid batteries in electric cars also threatens to increase heavy metal pollution. Batteries for a single electric car require about 1,000 pounds of lead, so the proliferation of electric cars using lead-acid batteries will greatly increase the demand for the noxious heavy metal, says Vitt, one of the graduate students on the LN2000 team. The greater the demand for lead, he adds, the greater the threat for pollution in mining, smelting, transporting, using and disposing of it.
Newer nickel-metal hydride and lithium-ion batteries offer better range and performance, but they are prohibitively expensive and potentially dangerous. As the amount of stored energy increases in these batteries, Hertzberg explains, they become more unstable and likely to explode in the event of an accident.
"Obviously in developing any new product you have new environmental concerns," counters Bogdanoff, "but these are old arguments that really aren't being made anymore. Electric cars, no matter what kind of battery you're using, are better than gas-powered cars."
Hertzberg is not so sure.
"The safe batteries are an ecological mess and can't go anywhere," he says. "The ones that can go somewhere are incredibly expensive and quite dangerous. You'd have to work really hard to hurt yourself with a liquid nitrogen vehicle. Liquid nitrogen isn't combustible, corrosive or toxic. It's just cold."
Ironically, Hertzberg says, the cold temperature is precisely what stalled previous efforts to develop a liquid nitrogen vehicle. Researchers have known for decades that pressure built up when liquid nitrogen is converted to a gas could power a car. But the UW group was the first to develop a heat exchanger system that doesn't freeze up when exposed to the nippy liquid nitrogen.
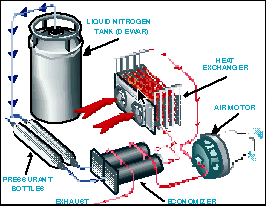
The LN2000's heat exchanger pulls liquid nitrogen from an insulated fuel tank through a series of aluminum tubing coils and specially designed pipes. Engine exhaust and outside air are circulated around the coils and pipes to gradually warm up the nitrogen from a minus 320 F liquid to an ambient-temperature gas.
"The heat exchanger is like the radiator of a car, but it acts in the opposite way," explains Bruckner. "Instead of using air to cool water, it uses air to boil liquid nitrogen into nitrogen gas."
The conversion from liquid to gas expands the volume of the nitrogen 700 times, building sufficient pressure to turn an air motor much like pressure from burning gasoline drives an internal combustion engine.
With a $360,000 U.S. Department of Energy grant, UW researchers and students have built a LN2000 prototype from a converted Grumman Kubvan mail truck. Aside from the Husky decals, the vehicle looks much like any other postal truck on the outside. But open up the back and one finds insulated tanks, piping, hoses and gauges more befitting a research laboratory than an automobile. Under the hood is a 15 horsepower air motor originally designed to power a winch for raising ship anchors. And instead of generating plumes of foul exhaust, the LN2000 emits cold nitrogen gas which freezes water vapor in the air to form small clouds behind the vehicle.
While the reliance of internal combustion engines on non-renewable fuels has prompted automotive engineers to make car motors more efficient over the years, air motors have had the luxury of remaining fabulously inefficent. As a result, the motor used in the LN2000 prototype gives gas guzzling new meaning by consuming about five gallons of nitrogen fuel per mile. Plus it musters a top speed of only 22 m.p.h. and chugs laboriously up hills.
"This motor operates at less than 20 percent of the efficiency we think is possible, which reduces power, performance and fuel economy," explains John Williams, a former graduate student who worked on the LN2000 project for his master's thesis. "We know we can do better."
Next Steps in Liquid Nitrogen Car Research
Abe Hertzberg: The Reluctant Idea Man
Send a letter to the editor at columns@u.washington.edu.